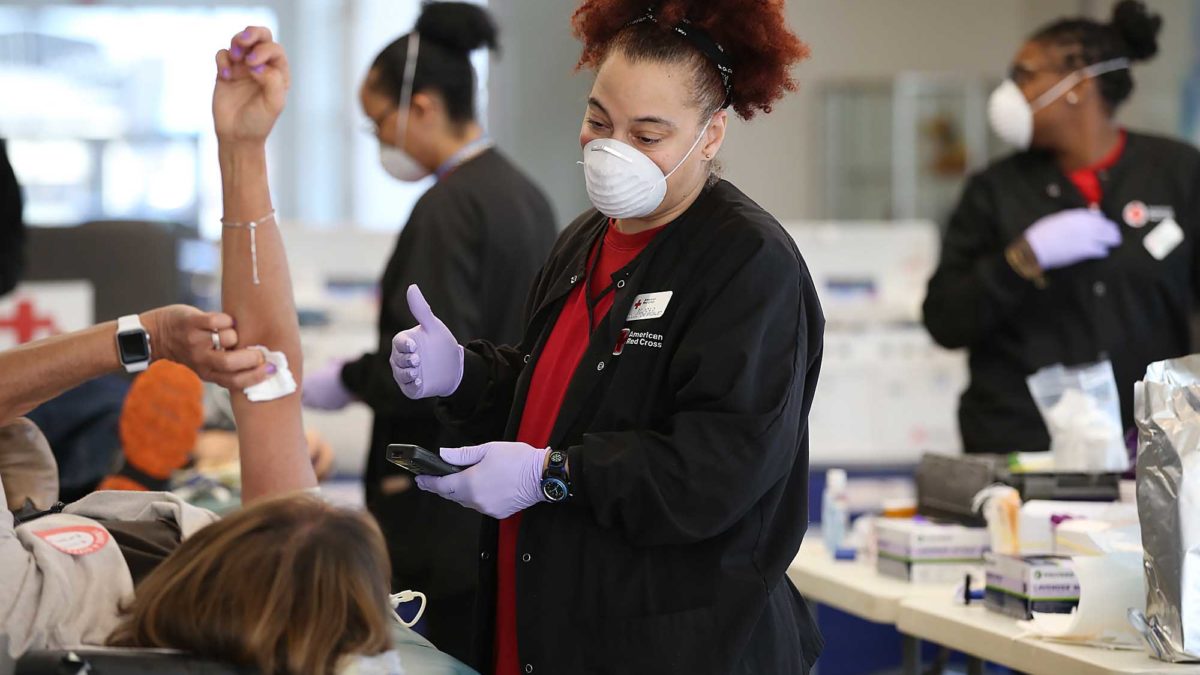
Antibody testing could help identify who can donate convalescent plasma for the treatment of Covid-19, according to the American Red Cross.
The American Red Cross said in a statement on Wednesday that it is working to implement antibody testing to help screen for recovered Covid-19 patients who could donate blood plasma to help ill patients fight the disease.
The idea is that convalescent plasma, the liquid part of blood, from recovered patients contains antibodies that could help strengthen the immune response of other patients still battling infection. Last week, the US Food and Drug Administration announced that patients who are fully recovered from Covid-19 for at least two weeks are encouraged to consider donating plasma.
While “thousands of potential donors” responded to that call, less than 10% initially met the FDA’s eligibility criteria to donate, according to the American Red Cross statement. The criteria include having a verified Covid-19 diagnosis and either being symptom free for at least 28 days prior to donation or symptom free for at least 14 days prior to donation while also having a negative Covid-19 test result.
“The Red Cross is currently reviewing and requesting additional information from individuals who have submitted donor information. However, this is a complex process as we work to ensure each potential donor is appropriately screened and has the proper documentation to ensure … every convalescent plasma product collected is safe for a patient battling Covid-19,” the American Red Cross said in its statement on Wednesday.
“We are encouraged that FDA approved Ortho Clinical Diagnostic’s Covid-19 Antibody Test through an Emergency Use Authorization last week. The Red Cross is working with our partner, Creative Testing Solutions (CTS), to implement this test in the near future,” the statement said in part. “This automated test has the potential for a high throughput, enabling the collection of convalescent plasma from the many donors who do not currently have a confirmed positive Covid-19 diagnosis, and need an antibody test to confirm their eligibility to participate in the convalescent plasma program.”
For now, recovered patients can contact their local blood or plasma collection center to schedule an appointment to donate. The FDA noted in its previous announcement that it has launched a new webpage with information on blood and plasma collection centers, and the American Red Cross also set up a website for interested donors.


Демо игровых автоматов предлагают уникальную возможность попробовать поиграть в казино, не тратя реальные деньги. Это идеальный способ испытать удачу, изучить игры и разработать стратегии без расхода средств.
Благодаря широкому выбору демо-слотов, каждый игрок найдет что-то по своему вкусу. От классических трехбарабанных слотов до современных видеослотов с крутейшей графикой и увлекательными бонусными раундами.
Играть в [url=https://lucky-slots.ru/klassicheskie-sloty/]классические слоты играть в игровые автоматы онлайн[/url] легко и удобно. Вам не нужно создавать аккаунт или делать депозит – просто выберите интересующую вас игру и начинайте вращать барабаны. Это отличная возможность попробовать разные стратегии ставок, изучить выигрышные комбинации и просто кайфануть в игру в казино.
Демо-режим также дает возможность вам сделать оценку отдачи игрового автомата и определить, подходит он вам или нет. Вы можете играть сколько угодно долго, не беспокоясь о своем бюджете.