US health agencies are working to assess whether the Johnson & Johnson Covid-19 vaccine is associated with a very small increased risk of rare blood clots, a federal official told CNN.
“The CDC and the FDA are taking these concerns about blood clots and the J&J vaccine seriously and are diligently assembling data,” the official said.
An expert outside the government who is familiar with the situation agreed that health officials are taking the matter seriously.
“The CDC is very concerned and they’re very working hard on this and monitoring this closely,” said the expert, who spoke on the condition of anonymity due to the sensitive nature of the issue.
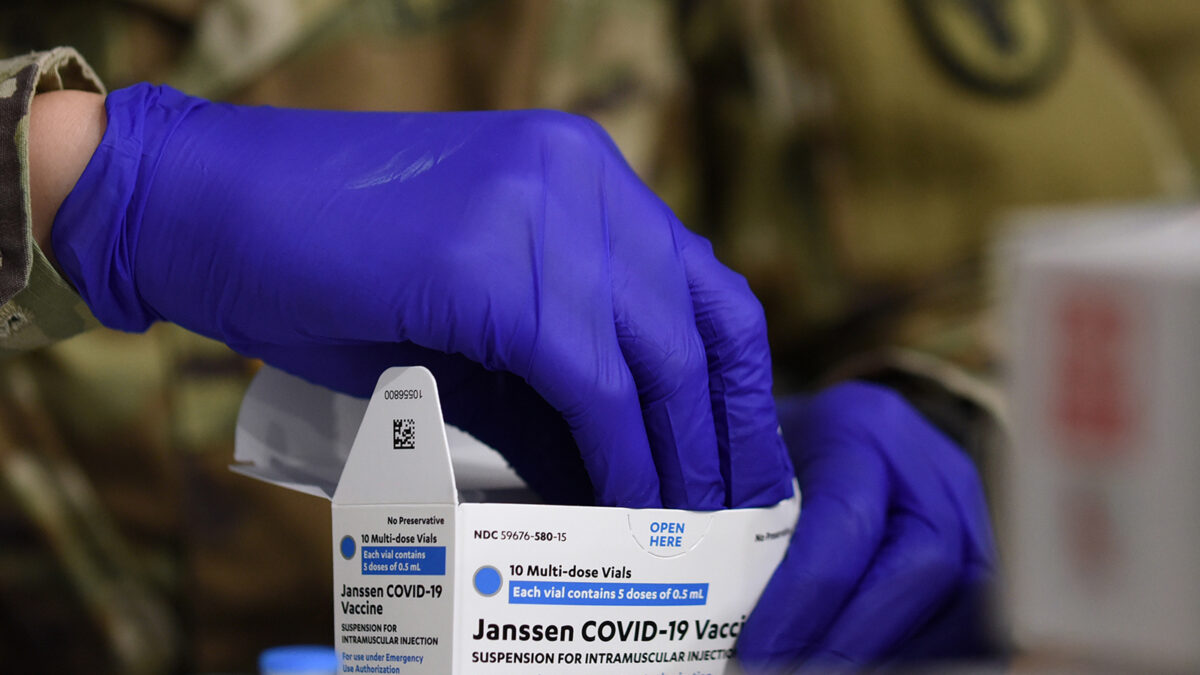
Cases so far: There have been “four serious cases of unusual blood clots” reported after people received the Johnson & Johnson vaccine, according to European health authorities.
Some of the types of blood clots observed are relatively common, such as deep vein thrombosis, so it wasn’t surprising that among roughly 20,000 participants who received the vaccine, some would experience those clots.
What made FDA scientists take note, however, is that in the trial, about the same number of people received a placebo — a shot of saline that does nothing — as received the vaccine. However, when comparing the two groups, more study participants developed clots after receiving the vaccine than the placebo.
The link is “not clear: Like their US counterparts, the European authorities say they’re still investigating these cases and that “it is currently not clear” whether there’s a causal association between the vaccine and the clots.


Really looking forward to reading more. Read more. Great blog.
PBN sites
We shall build a system of private blog network sites!
Pros of our private blog network:
We carry out everything SO THAT google DOES NOT realize that this A private blog network!!!
1- We purchase domains from various registrars
2- The principal site is hosted on a VPS server (VPS is high-speed hosting)
3- The remaining sites are on distinct hostings
4- We assign a individual Google account to each site with verification in Search Console.
5- We make websites on WordPress, we don’t utilize plugins with assisted by which malware penetrate and through which pages on your websites are created.
6- We do not reiterate templates and utilize only distinct text and pictures
We never work with website design; the client, if wanted, can then edit the websites to suit his wishes
Rolex watches
Understanding COSC Accreditation and Its Importance in Watchmaking
COSC Accreditation and its Strict Criteria
Controle Officiel Suisse des Chronometres, or the Controle Officiel Suisse des Chronometres, is the official Swiss testing agency that attests to the precision and accuracy of wristwatches. COSC accreditation is a sign of excellent craftsmanship and dependability in chronometry. Not all watch brands pursue COSC validation, such as Hublot, which instead adheres to its proprietary stringent criteria with movements like the UNICO, reaching comparable accuracy.
The Science of Precision Timekeeping
The central mechanism of a mechanized watch involves the spring, which supplies energy as it unwinds. This system, however, can be vulnerable to external factors that may impact its precision. COSC-validated movements undergo strict testing—over 15 days in various conditions (5 positions, 3 temperatures)—to ensure their resilience and reliability. The tests measure:
Mean daily rate precision between -4 and +6 seconds.
Mean variation, highest variation levels, and effects of thermal variations.
Why COSC Accreditation Matters
For timepiece aficionados and collectors, a COSC-validated timepiece isn’t just a piece of technology but a demonstration to lasting excellence and precision. It symbolizes a watch that:
Offers excellent reliability and accuracy.
Offers guarantee of superiority across the entire design of the timepiece.
Is likely to retain its worth more effectively, making it a sound choice.
Well-known Chronometer Manufacturers
Several famous manufacturers prioritize COSC certification for their watches, including Rolex, Omega, Breitling, and Longines, among others. Longines, for instance, offers collections like the Archive and Spirit, which feature COSC-validated mechanisms equipped with innovative substances like silicon balance suspensions to improve resilience and efficiency.
Historical Background and the Evolution of Timepieces
The idea of the timepiece originates back to the requirement for exact chronometry for navigational at sea, highlighted by John Harrison’s work in the eighteenth cent. Since the formal foundation of COSC in 1973, the validation has become a benchmark for judging the accuracy of high-end watches, sustaining a legacy of superiority in watchmaking.
Conclusion
Owning a COSC-validated watch is more than an visual selection; it’s a dedication to excellence and accuracy. For those appreciating precision above all, the COSC certification provides peace of thoughts, ensuring that each certified watch will function dependably under various conditions. Whether for individual contentment or as an investment, COSC-certified timepieces distinguish themselves in the world of horology, maintaining on a tradition of meticulous chronometry.
En Son Zamanın En Büyük Gözde Kumarhane Platformu: Casibom
Kumarhane oyunlarını sevenlerin artık duymuş olduğu Casibom, en son dönemde adından sıkça söz ettiren bir bahis ve kumarhane web sitesi haline geldi. Ülkemizdeki en başarılı casino platformlardan biri olarak tanınan Casibom’un haftalık olarak değişen açılış adresi, sektörde oldukça taze olmasına rağmen güvenilir ve kazandıran bir platform olarak tanınıyor.
Casibom, rakiplerini geride bırakıp eski kumarhane sitelerinin üstünlük sağlamayı başarmayı sürdürüyor. Bu alanda eski olmak önemli olsa da, katılımcılarla iletişim kurmak ve onlara ulaşmak da aynı kadar değerli. Bu aşamada, Casibom’un 7/24 yardım veren canlı olarak destek ekibi ile rahatça iletişime geçilebilir olması büyük önem taşıyan bir fayda getiriyor.
Hızlıca artan oyuncuların kitlesi ile dikkat çekici Casibom’un gerisindeki başarı faktörleri arasında, sadece ve yalnızca kumarhane ve canlı casino oyunları ile sınırlı kısıtlı olmayan geniş bir hizmet yelpazesi bulunuyor. Sporcular bahislerinde sunduğu geniş seçenekler ve yüksek oranlar, oyuncuları ilgisini çekmeyi başarmayı sürdürüyor.
Ayrıca, hem sporcular bahisleri hem de kumarhane oyunlar oyuncularına yönlendirilen sunulan yüksek yüzdeli avantajlı bonuslar da dikkat çekiyor. Bu nedenle, Casibom hızla piyasada iyi bir reklam başarısı elde ediyor ve önemli bir katılımcı kitlesi kazanıyor.
Casibom’un kazandıran promosyonları ve popülerliği ile birlikte, platforma üyelik hangi yollarla sağlanır sorusuna da değinmek gerekir. Casibom’a mobil cihazlarınızdan, bilgisayarlarınızdan veya tabletlerinizden tarayıcı üzerinden rahatça ulaşılabilir. Ayrıca, platformun mobil cihazlarla uyumlu olması da büyük önem taşıyan bir artı sunuyor, çünkü artık neredeyse herkesin bir akıllı telefonu var ve bu akıllı telefonlar üzerinden kolayca giriş sağlanabiliyor.
Taşınabilir cihazlarınızla bile yolda canlı iddialar alabilir ve müsabakaları gerçek zamanlı olarak izleyebilirsiniz. Ayrıca, Casibom’un mobil uyumlu olması, memleketimizde kumarhane ve casino gibi yerlerin kanuni olarak kapatılmasıyla birlikte bu tür platformlara erişimin önemli bir yolunu oluşturuyor.
Casibom’un güvenilir bir casino sitesi olması da önemlidir bir fayda sağlıyor. Belgeli bir platform olan Casibom, kesintisiz bir şekilde eğlence ve kazanç elde etme imkanı sunar.
Casibom’a üye olmak da son derece kolaydır. Herhangi bir belge gereksinimi olmadan ve ücret ödemeden platforma kolaylıkla abone olabilirsiniz. Ayrıca, web sitesi üzerinde para yatırma ve çekme işlemleri için de birçok farklı yöntem bulunmaktadır ve herhangi bir kesim ücreti talep edilmemektedir.
Ancak, Casibom’un güncel giriş adresini takip etmek de elzemdir. Çünkü canlı şans ve kumarhane web siteleri popüler olduğu için yalancı siteler ve dolandırıcılar da belirmektedir. Bu nedenle, Casibom’un sosyal medya hesaplarını ve güncel giriş adresini periyodik olarak kontrol etmek önemlidir.
Sonuç, Casibom hem emin hem de kazandıran bir kumarhane web sitesi olarak dikkat çekiyor. Yüksek promosyonları, geniş oyun alternatifleri ve kullanıcı dostu mobil uygulaması ile Casibom, oyun tutkunları için ideal bir platform getiriyor.